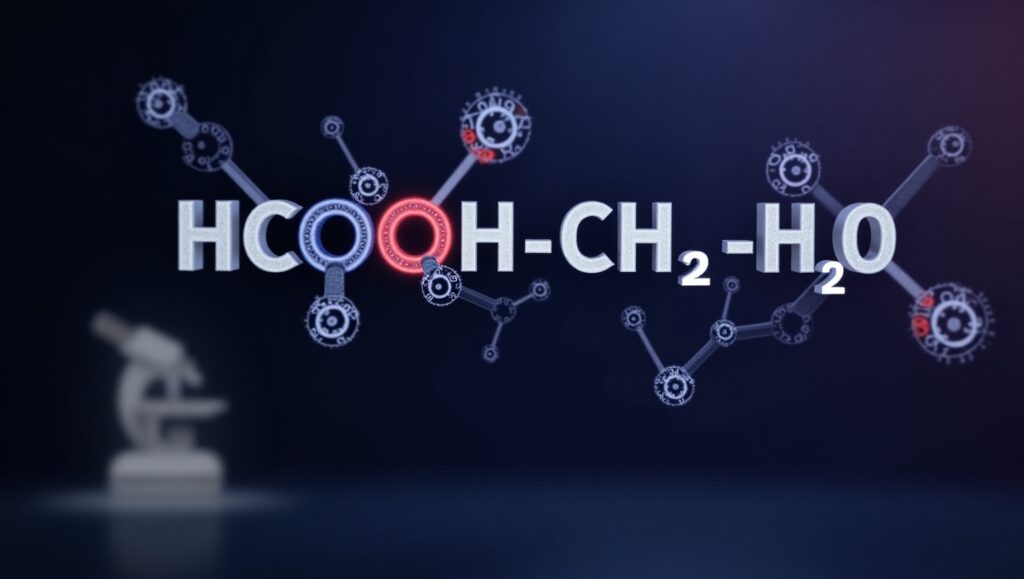
Chemistry plays a vital role in understanding how compounds interact with one another, and one such important reaction is the hydrolysis of methyl formate. The molecular formula HCOOCH₃ represents methyl formate, an ester derived from formic acid and methanol. When methyl formate reacts with water (H₂O), it undergoes hydrolysis, producing formic acid (HCOOH) and methanol (CH₃OH).
This reaction is a classic example of ester hydrolysis, commonly studied in organic chemistry.
What is Methyl Formate (HCOOCH₃)?
Methyl formate is the methyl ester of formic acid. It is a colorless liquid with a pleasant odor and is widely used in:
- The production of formamide (a precursor in pharmaceuticals and agrochemicals).
- Solvents for resins and coatings.
- Industrial processes as an intermediate.
Its chemical structure is:
HCOOCH₃ → H–C(=O)–O–CH₃
Hydrolysis Reaction of Methyl Formate
The general equation for the hydrolysis of methyl formate is:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
- Reactants:
- Methyl formate (ester)
- Water (H₂O)
- Products:
- Formic acid (HCOOH)
- Methanol (CH₃OH)
This is an ester hydrolysis reaction where the ester bond breaks down in the presence of water.
Mechanism of Hydrolysis
The hydrolysis of esters like methyl formate can occur in two major ways:
1. Acid-Catalyzed Hydrolysis
- In acidic conditions, the carbonyl oxygen of the ester is protonated.
- Water attacks the carbonyl carbon, leading to bond cleavage.
- This produces formic acid and methanol.
- The reaction is reversible under acidic conditions.
2. Base-Catalyzed Hydrolysis (Saponification)
- Hydroxide ion (OH⁻) attacks the carbonyl carbon.
- This breaks the ester linkage.
- The reaction produces formate ion (HCOO⁻) and methanol.
- Unlike acid hydrolysis, this reaction is irreversible.
Industrial Significance
The hydrolysis of methyl formate has several practical applications:
- Production of Formic Acid
Hydrolyzing methyl formate is one of the methods used to manufacture formic acid, which is widely applied in leather tanning, rubber production, and as a preservative. - Methanol Recovery
The reaction also yields methanol, which is used as a fuel, solvent, and raw material in plastics and chemical industries. - Synthetic Chemistry
Hydrolysis provides a simple way to convert esters into their parent acids and alcohols, making it essential in laboratory and industrial synthesis.
Conditions Affecting Hydrolysis
Several factors influence how fast and efficiently methyl formate hydrolyzes:
- pH level: Acidic or basic conditions accelerate hydrolysis.
- Temperature: Higher temperatures increase reaction rates.
- Catalysts: Strong acids (H₂SO₄) or bases (NaOH) are commonly used as catalysts.
- Solvent environment: The presence of polar solvents can stabilize intermediates, promoting faster hydrolysis.
Environmental and Safety Aspects
- Methyl formate is flammable and requires careful handling.
- Formic acid, one of the hydrolysis products, is corrosive and can cause skin burns.
- Proper storage, controlled reaction conditions, and use of safety equipment are essential in laboratories and industries.
Conclusion
The reaction HCOOCH₃ + H₂O → HCOOH + CH₃OH is a fundamental example of ester hydrolysis. Through this process, methyl formate breaks down into formic acid and methanol, both of which have significant industrial applications. Understanding this reaction not only strengthens concepts in organic chemistry but also highlights its importance in large-scale chemical manufacturing.
✅ Key Takeaway: The hydrolysis of methyl formate is an essential reaction that bridges academic learning with real-world industrial chemistry, demonstrating how simple organic reactions contribute to useful applications.